Electrochemistry
Our goal is to identify activity, selectivity and stability-determining properties of electrocatalysts and to achieve a comprehensive understanding of the solid-liquid interface under stationary and dynamic electrochemical reaction conditions. We study a variety of catalyst systems ranging from well-defined single crystal surfaces, to thin films, supported size and shape-controlled nanoparticles and industrially-relevant powder catalysts.
Electrochemistry and electrocatalytic reaction are highly important research fields as electrochemical technology allows progress with respect to the current most urgent societal and industrial challenges. For instance, we address the question of how to establish a CO2-neutral and sustainable power supply and the production of feedstock chemicals. Here, significant advances in the field of electro-mobility have been made thanks to the development of battery and fuel cell technologies. However, critical advances have not yet been achieved, such as the economic production of hydrogen and feedstock chemicals fuels from CO2, although, thanks to very active basic research efforts, those processes are now on their way to commercialization. In this respect, the development of highly stable and reliable electrocatalysts with appropriate catalytic activity and selectivity is one of the key points.
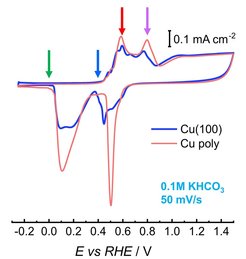
To overcome these challenges, it is essential to understand the evolution of the structure of these materials at the atomic scale under industrially-relevant conditions. Therefore, we are working on the development of tailored size-, shape-selected nanoparticle catalysts and nanostructured thin films either as model systems deposited on e.g. single crystal surfaces or as catalyst-support ensembles to be applied in e.g. electrolyzers and fuel cells. We apply colloidal electro and chemical synthesis techniques such as the inverse micelle encapsulation route, but also physical deposition techniques such as PVD and magnetron sputtering. In addition to monometallic electrocatalysts, we also investigate compositional effects either as alloys or mixed metal oxides or as core-shell structures.
The main electrochemical reactions that we are currently targeting are: CO2RR, HER, OER, N2RR and alcohol oxidation reactions. In all cases, our goal is to comprehensively investigate and identify the most important activity-/selectivity-/stability-determining properties and to achieve advanced mechanistic understanding. In addition to a variety of experimental techniques to perform a detailed physico-chemical characterization of the electrocatalysts, our toolbox comprises various in situ / operando microscopic and spectroscopic tools available in our laboratories and in synchrotron radiation facilities. Our main laboratory-based in situ and operando techniques include vibrational spectroscopy like electrochemical Raman and FTIR spectroscopy to access surface adsorbates under reaction conditions, or structural analysis using electrochemical (grazing-incidence) X-ray diffraction and X-ray absorption spectroscopy (XAS). Additionally, we apply quasi in situ XPS and NAP-XPS in electrochemical cells to thoroughly determine the surface chemistry and composition before and after electrocatalysis without any air exposure. We also perform operando electrochemical X-ray absorption spectroscopy in the soft X-ray regime as well as X-ray photoelectron spectroscopy at Bessy II, the synchrotron radiation facility in Berlin.
As the transformation of laboratory-based insights to practical application is essential to achieve a societal and industrial benefit, we are also working on industrially-relevant conditions like electrolyzer technology. Here, the most urgent question is to which extent the insights gained on model systems can be transferred to real devices. We would like to address if there are additional activity-/selectivity-/stability-determining properties which have to be considered when designing advanced electrocatalysts, or how do electrocatalysts respond under the different reaction conditions (e.g. higher current densities, temperatures, etc.) in terms of structure, morphology, and composition.
Group Members
Dr. Hyo Sang Jeonhyosang@fhi-berlin.mpg.de
Dr. Fabian Scholtenscholfry@fhi-berlin.mpg.de
Recent Publications
