Structure and Reactivity
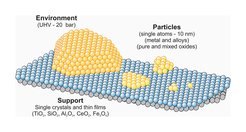
We study surface chemistry of oxide-supported metal and transition metal oxide nanoparticles focusing on the effect of the particle size, shape and the nature of oxide support on reactivity, particularly in CO2 hydrogenation reactions.
Mono- and multi-component particles, prepared for example by colloidal chemistry methods, are deposited onto planar oxide supports by dip coating, followed by oxygen plasma ligand removal and calcination-reduction treatments. In addition, metallic particles can be prepared by physical vapor deposition in vacuum.
In several UHV setups, we use a variety of surface science techniques such as scanning probe microscopy (SPM), Auger electron spectroscopy (AES), ultraviolet photoelectron spectroscopy (UPS), low energy ion scattering (LEIS), temperature programmed desorption (TPD), infrared reflection absorption spectroscopy (IRAS) (also in polarization-modulation mode, PM-IRAS), and X-ray photoelectron spectroscopy (XPS) in UHV as well as at near atmospheric pressures (NAP-XPS). In addition, the elementary steps of reactions are addressed by molecular beam (MB) studies in combination with IRAS. The catalytic activity of model planar catalysts under both, UHV-compatible and realistic conditions can be monitored by a gas chromatography and mass-spectrometry. The results on model catalysts are compared to the “real” systems prepared on high surface area supports to rationalize the structure-reactivity relationships.
Group Members
- 4114
- 4237
- 4235
- 4142
- 4666

Recent Publications
Former Group Photos



