Inorganic Chemistry Department
Department Profile
Hello!
On behalf of all members of the Department of Inorganic Chemistry I thank you for the interest in our activities. We are an interdisciplinary group between chemistry and physics working in catalysis science. Our core mission is to contribute to the functional understanding of heterogeneous catalysis. We use the standard model of the single crystal approach as our operational base and identify conceptual additions that are necessary to make the model operational in high performance catalysis. In this way we lay the bridges across the “gaps” in catalysis science denominated in the literature over the last two decades.
Our group is active at two locations namely in Berlin at the FHI and in Mühlheim/Ruhr at the MPI CEC (Chemical Energy Conversion). This originates from the dual function of the director as member of the collegium at the FHI in Berlin and as founding director at the MPI CEC.
One core family of reactions of interest in our department is oxidation. We study the reaction of molecular oxygen with activated (olefins) and non-activated (small alkanes) hydrocarbons. Another family of reactions deals with the reductive activation of CO2 and di-nitrogen. Finally, we study the generation of hydrogen through the oxidation of water to di-oxygen. In a broader context all our projects revolve around the characterisation of the reactivity of solid interfaces. This includes also electrodes for liquid phase reactions and in batteries.
We concentrate in our work on functional understanding. This requires the controlled and reproducible synthesis of our interfaces by preferably chemical methods to include the control of real structure in the samples. Then we perform a suite of in-situ reactivity studies of the geometric and electronic structure. We observe texture morphology and charge carrier transport as mesoscopic parameters and the local electronic structure as molecular parameters. As we always determine the reactivity during spectroscopic observation we aim at constructing structure-function relations founded on causal interrelations.
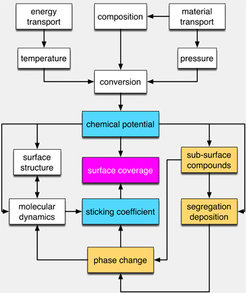
In our studies we find a dynamic response of the working catalyst to the local chemical potential of reactants that is defined by multiple variables that we have to control. The approach is indicated in Scheme 1.
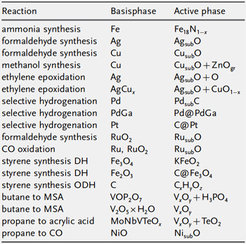
The generalization of these findings is attempted by studying an array of systems and reactions as indicated in the table. The actual systems under investigation are presented in the following description of ongoing work
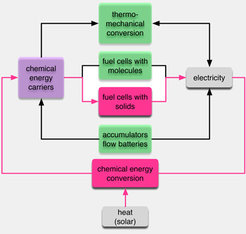
We bring this insight to bear in the context of chemical energy conversion. Here we study the use of chemical reduction reactions to store renewable primary electricity in molecular species known as “solar fuels”. Future sustainable energy systems need besides solar electricity also chemical energy carriers for multiple applications. We thus need to be able to freely convert all forms of energy carriers into each other which is currently not the case, at least on a technological scale as indicated in scheme 2.We thus concentrate on understanding the functional bottlenecks in water splitting. We do this mainly through electrochemistry noting that photochemical water splitting deals with the same elementary reaction steps. In photochemistry, we study various other oxidation reactions to arrive at fundamental insights into the requirements of such interfaces.
If we had renewable “green” hydrogen we would use it to reduce CO2 and nitrogen to methanol and ammonia as platform molecules with existing broad application. Only then we may envisage that such reactions would become economically attractive in competition to their fossil-based existing practice. To come closer to such targets we work on both sides of preparing large-scale implementations based on flue gas sources that need careful chemical investigation for trace components and fundamentally on finding the operational limits under conditions far from current practice for which industrial catalysts were optimized for decades. This work tests and demands robust fundamental understanding as extrapolation of our knowledge to widely differing chemical potentials of reactants will lead to structural responses of the catalyst as indicated in scheme 1 and thus to unexpected catalytic responses.
All this is only possible through our ability to device, implement and operate a broad range of analytical and synthetic methods. To this end we are most grateful to the many coworkers in our workshop facilities without their skillful and patient support we could not operate at all in our department. The such-attained problem-oriented competencies form the basis of our activities and thus for organizing the department in groups presenting their collaborative activities in the following material. We could not do our work without a significant operation at the BESSY synchrotron operated by HZB Berlin. We further have joined forces with BASF and TU Berlin in the joint laboratory BasCat dealing with industrial aspects of the feedstock challenge being an integrated problem in the ongoing energy transformation. We are further engaged in multiple cooperative activities with academic partners and with industry as indicated below. We do this predominantly to broaden our competence base and to get grounding with our understanding against practical application tests that we could not perform in an environment concentrated on fundamental science. We interact intensely with other groups within our institutes and within the MPG in order to stay connected with and to utilize results from related fundamental studies.
We welcome you to our activities and are grateful for your kind interest!
Robert Schlögl


