
CatLab - Catalysis Laboratory
Thin-Film Catalysts for one Sustainable Chemistry with Renewable Energies
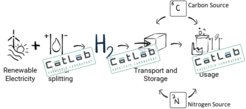
The urgently needed defossilisation of the energy system requires not only the drastic expansion of renewable electricity but also the development of alternative, sustainably produced material energy sources. A central task here is to develop new types of catalysts to ensure efficient conversion of chemical energy into electrical energy and vice versa - the core of CatLab.
Featured Publications
Teaming-up to take Hydrogen a Step Further
The Helmholtz-Zentrum Berlin (HZB), Fritz-Haber-Institut der Max-Planck-Gesellschaft (FHI), and Max-Planck-Institut für Chemische Energiekonversion (CEC) bundled their competencies in catalysis research, thin film, nanotechnology, and operando analysis, to give life to CatLab. In synergy with the Humboldt University of Berlin, the partners of UniSysCat and the industry, CatLab aims to generate pioneering knowledge and provide vital technological solutions for the establishment of a green hydrogen economy.
Enormous Potential for the Promotion of Young Scientists
An international pool of 100 experts works together in the framework of CatLab to develop cutting-edge catalytic systems using thin films and nanotechnology. This collaborative effort includes approximately 80 young scientists who work in interdisciplinary groups under the guidance of experienced senior scientists. In addition to providing training opportunities, CatLab fosters a spirit of collaboration among young scientists, enabling them to share ideas and establish partnerships within the project's scope.
Fostering Lasting Inter-Institutional Cooperation
The German Federal Ministry of Education and Research (BMBF) has generously funded CatLab as a 5-year project (December 2020 - November 2025). Our goal is to extend this collaboration beyond the funding period and establish a long-term partnership that drives groundbreaking research and innovation in the field of catalysis.
Highlights






















