Ångstrom-Depth Resolution with Chemical Specificity at the Liquid-Vapor Interface
Surfactants play an important role in every day life, for instance as major components in soaps. Since they feature hydrophilic and hydrophobic parts in their structure, they accumulate at water interfaces with air and can there influence the rate of evaporation of the solution or the efficiency with which gas molecules are taken up by the solution, a process that is for instance important for the incorporation of carbon dioxide into the oceans.
Elastically scattered electrons offer the clue
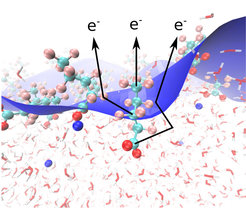
How surfactants arrange themselves at the interface of water with air is an intriguing question that has fascinated scientist for centuries, going back to Benjamin Franklin who noted the calming effect of cooking oil on the surface of water, and Agnes Pockels who did some of the first systematic experiments on the subject in the late 19th century. The question of the arrangement of surfactant molecules at the water-air interface is not easy to answer since a close look at the very skin of liquid water requires methods that hone in on the outer layers of water, where surfactant molecules are located in a layer with a thickness of only a few billionths of a meter.
A collaborative investigation of scientists from the Departments of Inorganic Chemistry, Molecular Physics and Theory at the Fritz Haber Institute in Berlin recently demonstrated a new method to address this problem, based on the elastic scattering of photoelectrons that are emitted upon irradiation of water – surfactant – vapor interface by X-rays. The surfactant that they studied was perfluorinated pentanoic acid, in which four of the five carbon atoms can be distinguished from each other in the C 1s (inner shell) core level photoelectron spectrum, and in particular the hydrophilic and the hydrophobic ends of the molecule can be distinguished from each other in the experiment. Perfluorinated pentanoic acid also belongs to the class of so-called “forever chemicals” that have recently moved into focus as prime pollutants in natural waters; these molecules are hard to remove and cause harm to the environment. The measurements were performed at the synchrotron radiation light sources BESSY-II in Berlin and SOLEIL near Paris at X-ray beamlines that allow to change the direction of the linear polarization of the X-rays. The angle between the direction of the polarization and the electron detector determines the intensity of the detected electron signal. The intensity distribution as a function of angle offers a clue on how many elastic “collisions” the electrons experienced on their way to the electron detector. Since water is a dense medium, electrons originating at those parts of the surfactant molecule that are immersed deeper into water will experience more elastic scattering than electrons that emerge from the parts of the molecule that stick out into air, which is much less dense than water. The experiments showed that the elastic scattering is sensitive enough to observe differences in the scattering from neighboring carbon atoms in the molecule, which are separated only by about one-ten billionth of a meter (0.1 nm).
Molecular Dynamics simulations provide the ruler
While the experiments qualitatively showed the expected orientation of the molecule, with the hydrophobic end pointing towards air and the hydrophilic end towards water, the experiments alone cannot quantify the average position of the molecule with respect to the water-air interface. This was possible using Molecular Dynamics simulations, that follow the trajectories of water and surfactant molecules over time and deliver a molecular scale “movie”. The average position of the surfactant relative to the sample surface can then be determined from many snapshots taken from that movie and compared to the elastic scattering data. It was found that there is an excellent agreement between the theoretical calculations and experimental data. This is encouraging for future measurements which will focus on the interplay of surfactant molecules with dissolved ions in water, a phenomenon that is present at the water-air interfaces of all natural systems, including oceans, rivers, and aqueous aerosol droplets.












