Unveiling Neutral Sulfur Formation in SF6 Under X-Ray Exposure
An international collaboration, including researchers from the Molecular Physics Department of the Fritz Haber Institute of the Max Planck Society, reveals the formation of neutral sulfur atoms during the decay of sulfur hexafluoride (SF6) under high-energy X-ray exposure. This study, utilizing advanced synchrotron-radiation techniques, provides new insights into the complex interactions of X-rays with matter, essential for scientific and technological advancements.
Key Aspects:
- Neutral Sulfur Formation: The study reveals that neutral sulfur atoms form during the decay of SF6 under high-energy X-ray exposure.
- Advanced Techniques: Developed advanced synchrotron-radiation techniques for this study, exposing molecules to hard X-rays and recording soft X-ray emissions - a challenging task due to the differing penetration depths of hard and soft X-rays in materials.
- Historical Context: Revisits a spectral anomaly first observed in 1978, providing new insights through modern technology and theory.
- Scientific and Practical Relevance: Enhances understanding of X-ray interactions with broad applications, including medical imaging and material science.
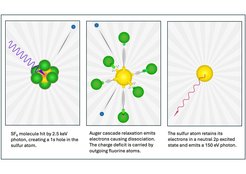
Understanding the interaction of X-rays with matter is fundamental to both scientific research and practical applications, including medical and technological advancements. These interactions involve intricate processes, including absorption, ionization, scattering, and the decay of excited states, resulting in the emission of electrons or photons.
In 1978, scientists Joseph Nordgren and Hans Ågren at Uppsala University discovered a spectral anomaly in sulfur hexafluoride (SF6). The nature of this anomaly remained a mystery for over four decades.
The team revisited this anomaly using advanced synchrotron-radiation techniques developed specifically for this study. In the experiment, SF6 molecules were exposed to hard X-rays that excited sulfur's innermost electron shell, initiating a cascade of electronic decays, leading to complete molecular dissociation and neutral-sulfur-atom formation.
The team, led by now emeritus professor Joseph Nordgren, Uppsala Universitet, Oksana Travnikova, CNRS, and Florian Trinter, FHI, employed synchrotron radiation to selectively study the soft X-ray emissions of intermediate states. Advanced theoretical calculations were also employed to interpret the soft X-ray emissions observed at specific energies, which could not be attributed to molecular fragments (e.g., sulfur bound to fluorine atoms) or charged sulfur states. These calculations provided crucial insights into the complex molecular response to deep inner-shell ionization of sulfur in SF6.
It was determined that, despite the ejection of multiple electrons during the decay cascade, neutral sulfur atoms were formed. This finding is surprising and unexpected given the strong electron-attracting nature of fluorine atoms. The research highlights the intricate electron density redistribution during the decay process and underscores the power of modern X-ray techniques in solving long-standing scientific puzzles.
This research not only resolves a decades-old mystery but also advances our understanding of molecular dynamics under X-ray exposure. The findings have significant implications for both scientific research and practical applications, demonstrating the enduring value of persistence and innovation in scientific discovery.












