Electrocatalysis
In order to make clean energy storage and conversion technologies, water electrolyzer, metal–air batteries, and CO2 electroreduction systems efficient and economically viable, electrocatalysts with significantly enhanced activity and selectivity for the desired products are required.
![Geometrical current density (left axis) and Faradaic efficiency for C2H4 (right axis) of Cu nanocube electrocatalysts after 1 h of electrochemical reaction at −1.0 V vs RHE in CO2-saturated 0.1 M KHCO3 for different plasma pre-treatments. [From Gao, Zegkinoglou et al., ACS Nano 11, 4825 (2017)].](/327995/original-1595871894.jpg?t=eyJ3aWR0aCI6MjQ2LCJvYmpfaWQiOjMyNzk5NX0%3D--2c3ad5aa640a5102e953b61e75a030031f7e87fa)
Our group focuses on the development and fundamental understanding of high-performance, stable catalysts for electrochemical reactions of technological interest, such as the oxygen reduction reaction (ORR), hydrogen evolution reaction (HER), oxygen evolution reaction (OER), CO2 electrochemical reduction reaction (CO2 RR) and N2 reduction reaction (N2RR).
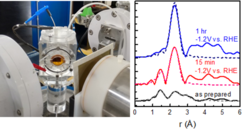
The goal of our research is to develop advanced nanoscale catalysts by systemically varying their size, morphology, chemical state and electronic properties. By using advanced operando and in situ techniques, such as X-ray photoelectron spectroscopy (XPS), X-ray absorption spectroscopy (XAS), nuclear resonant inelastic X-ray scattering (NRIXS), electrochemical atomic force microscopy (EC-AFM) and scanning tunneling microscopy (EC-STM), as well as liquid scanning electron (L-SEM) and liquid transmission electron microscopy (L-TEM), differential electrochemical mass spectrometry (DEMS), and electrochemical Raman and Fourier-transform infrared (FTIR) spectroscopy, we gain insight into the parameters affecting the activity and selectivity of electrochemical reaction processes.
Particularly in focus are in situ and operando spectroscopic techniques which provide insights into the electronic structure, atomic coordination and lattice vibration dynamics of the electrocatalysts under conditions which are similar to those in an operating electrochemical cell. For example, our group operates an XPS setup which is connected to an in situ electrochemical cell. The elemental composition, chemical state, and surface adsorbate species of the electrocatalysts are determined by XPS immediately after the electrocatalytic reaction, upon transferring the sample in a conrolled atmosphere from the electrochemical cell to the XPS analysis chamber.
![Summary of hydrocarbon selectivity of plasma-treated Cu foils for CO2 electroreduction as a function of the pre-treatment of Cu-foils. [From Mistry et al., Nat. Commun. 7, 12123 (2016)].](/327961/original-1595871894.jpg?t=eyJ3aWR0aCI6MjQ2LCJvYmpfaWQiOjMyNzk2MX0%3D--20c0cee3ee1705644e679cc7c23b302e4b618525)
For determining the chemical state and atomic coordination number of nanoscale electrocatalysts, operando XAFS studies are performed by our group at synchrotron facilities on a regular basis. By acquiring X-ray fluorescence spectra from an actual electrocatalyst while an electrochemical reaction is in progress, reaction-induced changes on the morphology and chemical state of the nanostructures can be monitored as they happen. This in turn allows us to correlate them with the activity and selectivity of the catalyst.
The effect of mild plasma pretreatments on the morphology and oxidation state of metallic surfaces and the consecutive enhancement of their electrocatalytic performance is also being investigated in our group. For example, in the case of defect-rich copper catalysts, it was shown that their catalytic properties for the electrochemical reduction of CO2 to C2-C3 products were significantly improved upon O2 plasma treatment.
Besides the morphology, the chemical state of a nanocatalyst before and, most importantly, during an electrochemical reaction can be also tuned by mild plasma pre-treatments. This can help improve the activity and selectivity of the catalyst, as was demonstrated in the case of a Cu nanocube system for the electrocatalytic reduction of CO2 to hydrocarbons and alcohols. Treatment of the catalyst’s surface with O2 plasma prior to the electrocatalytic reaction lead to the stabilization of surface and subsurface oxygen species during the reaction, which results in lower overpotentials and higher ethylene and ethanol selectivity.
![Experimental (left) and theoretical (right) Fe-projected vibrational density of states in pentlandite obtained from NRIXS and DFT calculations, respectively, during hydrogen evolution. The location of adsorbed H in the lattice is dependent on the applied potential and the amount of S vacancies. [From Zegkinoglou et al., J. Am. Chem. Soc.139, 14360 (2017)].](/328033/original-1595871894.jpg?t=eyJ3aWR0aCI6MjQ2LCJvYmpfaWQiOjMyODAzM30%3D--14e679863125b496ad422e18d2de958422a41cb5)
Besides XPS and EXAFS, operando nuclear resonant inelastic X-ray scattering (NRIXS) studies can also contribute to the understanding of the factors determining the performance of electrocatalysts. NRIXS probes the lattice vibrations of Fe-based catalytic materials, providing the phonon density of states as well as values of several thermodynamic properties during the electrochemical reaction. In addition, it can provide new knowledge on temperature-dependent atomic order-disorder transitions, structural phase transitions involving soft phonon modes of NPs, and the physical phenomena underlying electrical and thermal conductivity, heat capacity, vibrational entropy, electron-phonon coupling, Debye temperature etc.
Combined with first principles DFT calculations conducted by collaborating groups, the phonon density of states which is obtained directly from the NRIXS spectra can provide insight into changes in the morphology of the catalyst during the reaction (e.g. alloying or segregation) and give information about the adsorption mechanism of reactants or intermediate products which affect the reaction mechanism. This was, for example, demonstrated on pentlandite (Fe4.5Ni4.5S8) powders used as catalysts for the hydrogen evolution reaction. Operando NRIXS studies, performed at the synchrotron (APS, Argonne) on pentlandite electrodes, revealed a change of the atomic sites which are occupied by H atoms during the reaction as a function of the applied potential due to gradual occupation of the initially existing sulfur vacancies.
![Geometrical current density (left axis) and Faradaic efficiency for C2H4 (right axis) of Cu nanocube electrocatalysts after 1 h of electrochemical reaction at −1.0 V vs RHE in CO2-saturated 0.1 M KHCO3 for different plasma pre-treatments. [From Gao, Zegkinoglou et al., ACS Nano 11, 4825 (2017)]. Geometrical current density (left axis) and Faradaic efficiency for C2H4 (right axis) of Cu nanocube electrocatalysts after 1 h of electrochemical reaction at −1.0 V vs RHE in CO2-saturated 0.1 M KHCO3 for different plasma pre-treatments. [From Gao, Zegkinoglou et al., ACS Nano 11, 4825 (2017)].](/327995/original-1595871894.jpg?t=eyJkb19ub3RfdHJhbnNmb3JtIjp0cnVlLCJmaWxlX2V4dGVuc2lvbiI6ImpwZyIsIm9ial9pZCI6MzI3OTk1fQ%3D%3D--e32616c59128c23134b75d29e76f4210b5cdb0e3)

![Summary of hydrocarbon selectivity of plasma-treated Cu foils for CO2 electroreduction as a function of the pre-treatment of Cu-foils. [From Mistry et al., Nat. Commun. 7, 12123 (2016)]. Summary of hydrocarbon selectivity of plasma-treated Cu foils for CO2 electroreduction as a function of the pre-treatment of Cu-foils. [From Mistry et al., Nat. Commun. 7, 12123 (2016)].](/327961/original-1595871894.jpg?t=eyJkb19ub3RfdHJhbnNmb3JtIjp0cnVlLCJmaWxlX2V4dGVuc2lvbiI6ImpwZyIsIm9ial9pZCI6MzI3OTYxfQ%3D%3D--9d29b4be954d76a66e184c6334b7b8660bc9cb8d)
![Experimental (left) and theoretical (right) Fe-projected vibrational density of states in pentlandite obtained from NRIXS and DFT calculations, respectively, during hydrogen evolution. The location of adsorbed H in the lattice is dependent on the applied potential and the amount of S vacancies. [From Zegkinoglou et al., J. Am. Chem. Soc.139, 14360 (2017)]. Experimental (left) and theoretical (right) Fe-projected vibrational density of states in pentlandite obtained from NRIXS and DFT calculations, respectively, during hydrogen evolution. The location of adsorbed H in the lattice is dependent on the applied potential and the amount of S vacancies. [From Zegkinoglou et al., J. Am. Chem. Soc.139, 14360 (2017)].](/328033/original-1595871894.jpg?t=eyJkb19ub3RfdHJhbnNmb3JtIjp0cnVlLCJmaWxlX2V4dGVuc2lvbiI6ImpwZyIsIm9ial9pZCI6MzI4MDMzfQ%3D%3D--604f38bb9c81d78a99ee4118c65723ca2568b559)