Nanostructure Synthesis and Surface Modification
Colloidal chemistry
Size- and shape-selected metallic, monodisperse nanoparticles (NPs) are synthesized by using a colloidal synthesis method known as inverse micelle encapsulation technique. In this method, poly(styrene-b-2-vinyl pyridine) diblock copolymers consisting of a polar head (P2VP) and a non-polar tail (PS) are dissolved in a non-polar solvent (usually toluene) to form inverse micelles.
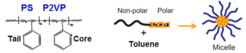
The micellar cages are then filled with metals by adding metal salts to the polymer solution. The resulting NPs are deposited by dip-coating on a planar support, forming a (sub)monolayer of hexagonally arranged model nanocatalysts. Alternatively, the NP solution can be attached to high-surface area supports (e.g. powders) by impregnation methods to yield real catalysts for reactivity studies.
![Deposition of NPs and subsequent plasma treatment for polymer removal: on planar supports (left) for model catalyst preparation, and on powders (right) for real catalyst preparation. [From Roldan Cuenya, Acc. Chem. Res. 46, 1682 (2013)].](/327600/original-1591111927.jpg?t=eyJ3aWR0aCI6MjQ2LCJvYmpfaWQiOjMyNzYwMH0%3D--a0601c0e3f2f4590b2ac2f80f606db7ec5e3818e)
The size and shape of the NPs can be tuned by varying either the molecular weight of the P2VP polymer core, or the atomic ratio of the metal salt to P2VP (metal loading). The interparticle distance can be also tuned by varying the molecular weight of the PS polymer tail. The final step of the synthesis procedure involves removal of the polymer by employing an O2 plasma treatment.
Plasma treatment of surfaces with various gases (O2, H2, N2, Ar) is also employed to modify their morphology and chemical state. By tuning the porosity and roughness (and thus the surface area) of catalytically active metal foils, a significant increase of the number of low-coordinated atomic sites can be achieved, leading to enhanced catalytic activity.

![Deposition of NPs and subsequent plasma treatment for polymer removal: on planar supports (left) for model catalyst preparation, and on powders (right) for real catalyst preparation. [From Roldan Cuenya, Acc. Chem. Res. 46, 1682 (2013)]. Deposition of NPs and subsequent plasma treatment for polymer removal: on planar supports (left) for model catalyst preparation, and on powders (right) for real catalyst preparation. [From Roldan Cuenya, Acc. Chem. Res. 46, 1682 (2013)].](/327600/original-1591111927.jpg?t=eyJ3aWR0aCI6ODQ4LCJmaWxlX2V4dGVuc2lvbiI6ImpwZyIsIm9ial9pZCI6MzI3NjAwfQ%3D%3D--9053da01a90ea8f0bb0b2f7402d1e745d3859254)