
Thermal catalysis
The performance of metallic nanoparticle catalysts for heterogeneously catalyzed reactions depends sensitively on the particle size, morphology and chemical state, as well as on the interparticle distance. These parameters can change significantly as a function of temperature, pressure and reactant flow during the catalytic reaction. Furthermore, interactions of the NPs with the support and the reactants (adsorbate effect) often result in a complex behavior which differs significantly from that of bulk materials or nanoparticles in vacuum. In the case of bimetallic systems, alloying and adsorbate-mediated segregation phenomena further complicate the situation, because they modify the elemental composition of the catalytically active surface.
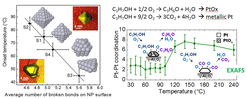
![Evolution of the 1st nearest neighbor Pt-Pt coordination number as a function of the H/Pt surface ratio extracted from EXAFS measurements on Pt22 clusters on γ-Al2O3 . [Mistry et al. ChemCatChem 6 (2014) 348; Behafarid et al. PCCP 14 (2012) 11766; PRB 84 (2011) 245438].](/327783/original-1595871124.jpg?t=eyJ3aWR0aCI6MjQ2LCJvYmpfaWQiOjMyNzc4M30%3D--de150d5f25b50a278079edf059e6954007d4363a)
An example of shape-dependent catalytic activity is shown on the right. We could find a correlation between the number of low-coordinated atoms (or broken bonds) at the surface of Pt NPs supported on γ-Al2O3 and the onset temperature for the partial oxidation of 2-propanol to acetone. By combining advanced microscopy (STM, TEM) and spectroscopy methods (XAS, MS) we were able to determine that the NPs were oxidized while acetone was being produced below ~100°C and remain metallic within the total oxidation reaction regime when CO2 was generated.
![Cu (red) and Ni (blue) atomic percentages extracted from in situ NAP-XPS measurements carried out on bimetallic CuNi NPs used in catalytic CO2 hydrogenation. The insets show AFM images of the sample before and after the annealing (scale bar: 400 nm), as well as atomic models of the NPs. [From Pielsticker et al., J. Phys. Chem. B, 2017, DOI: 10.1021/acs.jpcb.7b06984].](/327828/original-1595870787.jpg?t=eyJ3aWR0aCI6MjQ2LCJvYmpfaWQiOjMyNzgyOH0%3D--7162172834d89d2ae0cf0592c52078ad5ced1bc8)
The evolution of the nanoparticle shape can also be monitored as a function of the gaseous environment under realistic reaction conditions via XAS. Below is a representation of a 2D to 3D shape transformation observed for Pt NPs(~0.8 nm)/γ-Al2O3 (Pt22) as a function of increasing hydrogen coverage to a maximum pressure of 21 bar. The experimental data are displayed together with a theory reference for Pt13 clusters on Al2O3 [Mager-Maury et al. Chem.Cat.Chem. 3 (2011) 200].
Our group also employs near-ambient pressure (NAP-) XPS methods, both in our lab-based NAP-XPS setup, and at synchrotron facilities to investigate nanoparticle catalysts in reactive gas environments. Current projects include the study of CuNi, CuZn and NiGa catalytic systems for CO2 hydrogenation applications, as well as of NiCo systems for the catalytic partial oxidation of methane.

![Evolution of the 1st nearest neighbor Pt-Pt coordination number as a function of the H/Pt surface ratio extracted from EXAFS measurements on Pt22 clusters on γ-Al2O3 . [Mistry et al. ChemCatChem 6 (2014) 348; Behafarid et al. PCCP 14 (2012) 11766; PRB 84 (2011) 245438]. Evolution of the 1st nearest neighbor Pt-Pt coordination number as a function of the H/Pt surface ratio extracted from EXAFS measurements on Pt22 clusters on γ-Al2O3 . [Mistry et al. ChemCatChem 6 (2014) 348; Behafarid et al. PCCP 14 (2012) 11766; PRB 84 (2011) 245438].](/327783/original-1595871124.jpg?t=eyJkb19ub3RfdHJhbnNmb3JtIjp0cnVlLCJmaWxlX2V4dGVuc2lvbiI6ImpwZyIsIm9ial9pZCI6MzI3NzgzfQ%3D%3D--c897a64eda17a7f4c816ac92ffc04bbca5a621f1)
![Cu (red) and Ni (blue) atomic percentages extracted from in situ NAP-XPS measurements carried out on bimetallic CuNi NPs used in catalytic CO2 hydrogenation. The insets show AFM images of the sample before and after the annealing (scale bar: 400 nm), as well as atomic models of the NPs. [From Pielsticker et al., J. Phys. Chem. B, 2017, DOI: 10.1021/acs.jpcb.7b06984]. Cu (red) and Ni (blue) atomic percentages extracted from in situ NAP-XPS measurements carried out on bimetallic CuNi NPs used in catalytic CO2 hydrogenation. The insets show AFM images of the sample before and after the annealing (scale bar: 400 nm), as well as atomic models of the NPs. [From Pielsticker et al., J. Phys. Chem. B, 2017, DOI: 10.1021/acs.jpcb.7b06984].](/327828/original-1595870787.jpg?t=eyJkb19ub3RfdHJhbnNmb3JtIjp0cnVlLCJmaWxlX2V4dGVuc2lvbiI6ImpwZyIsIm9ial9pZCI6MzI3ODI4fQ%3D%3D--c2ef616f8d59b7e32021d932289365040c8536d7)