Benzene to Aniline
Li He, Matus Stredasnky, Liseth Correa Duarte, Walid Hetaba
Layered double hydroxides (LDH), represent a broad class of inorganic lamellar compounds with a high versatility in composition1. The two dimension lamellar compounds presents an increase potential during the last decades in the large range of technological applications2. The FHI research focuses on the investigation of supported metal-based catalyst systems of NiCuAl LDH by employing the coprecipitation method. The characterization of synthesized NiCuAl catalysts aims at the identification of essential catalyst features for synthesis of aniline (C6H5NH2) from benzene by a direct amination process.
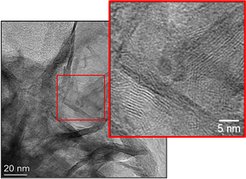
The different synthesis parameters, such as, aging time, washing method, synthesis temperature, pH, etc. have been investigated, in order to synthesize good crystal structure of LDH. The formation of stacked layers in the synthesis can be clearly determined in LDH with TEM images (Figure 1). Furthermore, the study of the activation procedures, involving a decomposition by heating procedure followed by reduction step in order to obtain a Cu/Ni nanoparticle finely and uniformly dispersed over alumina substrate. It has been found that the lower decomposition temperature (e.g., 290 °C) and the increase of Cu content in pristine LDH could effectively promote the Ni reducibility. More available characterization methods involved including in-situ XRD, synchrotron based spectroscopy like XPS/NEXAFS, elemental analysis as well as and high resolution electron microscopy.
The activated materials with different metal content were further tested for benzene direct amination to aniline, and reverse water gas shift reaction (rWGS) in Bascat.
1. Bukhtiyarova, M. V. A review on effect of synthesis conditions on the formation of layered double hydroxides. J. Solid State Chem. 269, 494–506 (2019).
2. D. G. Evans and R. C. T. Slade, “Structural aspects of layered double hydroxides,” Struct. Bond., (2005).
