In-situ Thermal Analysis
Dr. Andrey Tarasov
Recent discoveries in heterogeneous catalysis on academic and industrial field have largely been accelerated by the advances in analytical tools and wide application of complementary in-situ methods. To monitor the catalyst ́s properties on the fly under conditions of catalytic process will always be not a trivial task. However it always be a direct approach for mechanistic studies of heterogeneous solid-gas system which provides experimental evidences on the active state of the working catalyst.1 Wide technical realization and flexibility of thermal methods of analysis make it a very powerful tool for in-situ characterization of solid catalysts. In-situ thermal analysis has been employed in different process reactions of high industrial relevance. The research focuses on i) quantification of adsorbates under reaction conditions and characterization of catalytically formed adlayer2 ii) thermochemical characterization of active oxygen surface species in selective oxidation reactions (Fig. 1). iii) measurement of the heat effects of reversible and irreversible phase transitions in solid catalyst and its effect on the reactivity in oxidative dehydrogenation process (Fig. 2).
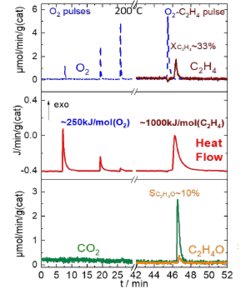
Ethylene epoxidation over silver catalyst
Alternating O2/C2H4 pulse sequence provides thermo-chemical information of the silver-oxygen interaction by introducing oxygen pulses to the catalyst Ag/αAl2O3 after quenched steady state. Silver surface interacts with oxygen with strong exothermicity, which allows to determine the formation enthalpy of Ag-Ox. Oxygen deficiency could be refiled with successive pulses. Interaction is neither accompanied with O2 desorption, nor with evolution of any gaseous products implying formation of oxygen rich silver layer. To probe the reactivity of the reabsorbed oxygen time delayed C2H4 pulse was introduced after saturation with O2. The C2H4 consumes preabsorbed/prestored oxygen and reacts towards products with certain heat effect. This procedure tends to answer the question how selective are the prestored oxygen species
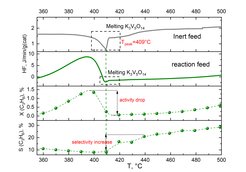
Oxidative dehydrogenation of propane on supported liquid phase catalyst
Aerosil supported Alkali based vanadates were investigated under reaction conditions in temperature range 300-500°C. It was shown that the melting of the supported phase in inert feed correlates directly with the drop of activity and increase of selectivity under reaction conditions. This phenomena was shown have a general character for K, Cs and Rb vanadates. Molten catalyst blocks the pores and reduces the BET surface area resulting in a significant performance change. In addition the stability of the phase composition during reaction could be considered as main reason of the change in melting behavior in sequential DSC runs.
[1] M. Y. Sinev, V. Y. Bychkov, Kinet. and Catal. 40 (1999) 819-835.
[2] A. V. Tarasov, F. Seitz, R. Schlögl, E. Frei, ACS Catal. 9 (2019) 5537-5544

