Liquid Jet Photoelectron Spectroscopy (LJ-PES)
Research in the Winter group focuses on the electronic structure interactions in liquid water and aqueous solutions of common electrolytes, many organic and inorganic solute molecules, including amino acids, DNA, and even from (transition) metal nanoparticles dispersed in water. In our experiments we apply Liquid-microJet PhotoElectron Spectroscopy (LJ-PES), mostly in conjunction with monochromatized soft X-rays from synchrotron light facilities, BESSY, DESY, SOLEIL, or SPring8.
Electronic structure information is intimately connected with the fundamental properties of liquid water and aqueous solutions. For example, we measure lowest ionization energies from the aqueous solvent and embedded solutes which directly relate to chemical reactivity. On the other hand, core-level PES from aqueous solutions, often measured at some ionization threshold (opening multiple unique electronic relaxation channels, local and non-local), reveals information on the local chemical environment, ion pairing, charge state, dynamical hydration configuration, hydrogen bond strength, intermolecular charge and energy transfers, ultrafast nuclear dynamics and chemical transformations, the density profile of solute species across the aqueous solution–vacuum interface, and interfacial and bulk-solution chemical equilibria. Moreover, experimental Photoelectron Angular Distributions (PAD) reveal the effect of hydrogen bonding on the orbital character of aqueous species, where we refer to the PADs measured in the dipolar plane, from which the β2 anisotropy parameter is accessed.
Terrestrial organisms display differentiated responses to chiral molecules encountered in their environment (pheromones, odors, pharmaceuticals) and in many cases these interactions may incorporate (partial) solvation effects. This generates interest in fundamental investigations of condensed-phase chirality. We plan to extend the ability of LJ-PES to probe chirality in the aqueous phase by introducing PhotoElectron Circular Dichroism (PECD) to LJ-PES. Photoionization of a chiral molecule by Circularly Polarized Light (CPL) introduces a forward – backward asymmetry in the flux of the ejected electrons with respect to the light propagation direction. The difference seen between left- and right-CPL-driven photoelectron emission, for a given enantiomer and given detection angle, defines the actual PECD. The PECD effect may be expected to be enhanced or diminished in the aqueous phase in comparison to isolated molecule cases, revealing the role of hydration in modulating molecular chirality and, potentially, stereoselective reactions. Our group also has a strong interest in advancing the LJ-PES technique by developing unique and efficient electron detectors compatible with liquid jets, and we also work on novel designs of flat microjets; the latter being indispensable for PAD measurements and providing means to induce and study slow chemical reactions by crossing two cylindrical jets of different solutions.
Some examples of our current research focus are presented below:
Water is one of the most ubiquitous molecules throughout natural sciences, yet the seemingly simple molecule also constitutes one of the most challenging systems to understand. We study the very fundamental properties of liquid water, from hydrogen-bond networks to energy, charge and proton transfer processes, largely by exploring the autoionization processes following core-level ionization.
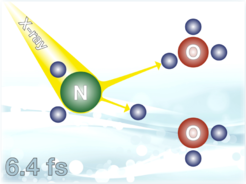
The most central aspect is the observation of a high-kinetic energy signal in the Auger autoionization spectra of liquid water (as well from other hydrogen bonding molecules, such as NH3 (aq.)), which is a signature of a special non-local relaxation process (ICD) involving two neighboring hydrogen-bonded molecules. In the case of NH3 (aq.) a complete ultrafast chemical transformation to form H3O+ from core-level ionized NH3.
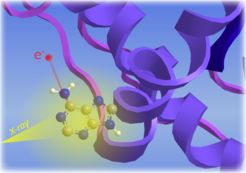
The liquid microjet technique opens a window for investigating electronic structure properties of biomolecules in their natural aqueous environment. This enables us to study how the bonding networks of biomolecules respond to environment changes, such as pH or concentration, and to investigate complex processes such as radiation damage of DNA or Adenosine Triphosphate (ATP) hydrolysis.
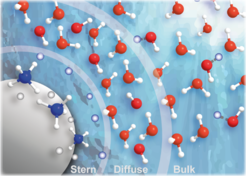
Aqueous-phase nanoparticles: We study (a) the intermediate metal-oxo oligomers that lead to nanoparticle (NP) formation in aqueous solutions. Second (b) focus is on the nature of water interaction (molecular versus dissociative adsorption) with the NP surface, and the dependence on pH. Our studies also aim at characterizing the electric double layer surrounding the aqueous-phase NPs.
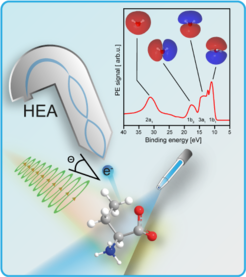
Chiral species exist in two non-superimposable mirror-image structures that respond differently towards circularly polarized light; in photoionization by circularly polarized light this leads to a strong forward – backward asymmetry of the ejected electrons. We apply PhotoElectron Circular Dichroism (PECD) to explore how a liquid (and particular aqueous) environment affects the dichroic properties of chiral solutes, and how intermolecular interactions with the solvent can extend the chiral system.
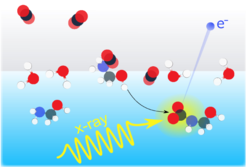
Gas – liquid interfacial dynamics and reactivity are highly interesting from fundamental and from atmospheric research perspectives; we focus in particular on energy and matter exchange between the two phases during their interactions. We employ different experimental setups (crossed-beam scattering or gas streaming around cylindrical jets) to vary the conditions for the interfacial dynamics. As an example, we study how CO2 can be captured by a water/ethanol-amine mixture with which the CO2 molecules react at the solution interface.

Liquid ammonia is a particularly interesting hydrogen-bonding counterpart to liquid water but LJ-PES must be conducted at cryogenic temperature. The first valence and core-level PES measurements from liquid ammonia were recently demonstrated, using a novel cryogenic liquid jet developed by the Jungwirth (Prague), Bradforth (USC) groups, and FHI. Ongoing work focusses on the energetics of the ammoniated electron (at low concentration) and on the liquid ammonia-to-metal transition upon addition of large-enough amount of alkali metal. The photograph shows the glass reservoir containing a metal – liquid ammonia solution with the characteristic blue color of solvated electrons.






