Experimental methods
LJ-PES Experimental Setup
The three main components of our new setup “EASI” (Electronic structure from Aqueous Solution and Interfaces) are a SCIENTA HiPP3 electron analyzer, a unique architecture of the vacuum chamber, and novel liquid jet designs. Features include the capability to detect:
Photoelectron Angular Distributions, PADs, in the dipolar plane
Photoelectrons emitted perpendicular to the dipole plane, in the direction of the light propagation (uniquely suited for LJ-PECD measurements)
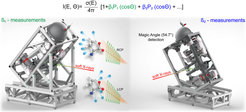
Also other orientations are possible with our new EASI setup as shown below

Molecular Beam - (Flat) Jet Interaction Chamber
Experiment in preparation. The setup is currently used along with the development of novel aqueous-solution flat jets, including mixing of two cylindrical microjets, each carrying a different aqueous solution. The example below shows the luminescence observed when mixing a luminol aqueous solution microjet with a hydrogen-peroxide aqueous solution jet. Specific aspects of these pioneering studies include (i) optical determination of film thickness, (ii) spectroscopic measurement of jet temperature, and (iii) characterization of turbulent and diffusive mixing of two liquids.

Aqueous-Phase Solutes Studied Include:
- Hydrated electron
- Water’s ionization products, OH-, H3O+
- Simple electrolytes
- Hydrogen-bonding molecules, H2O2, NH3
- Nanoparticles
- Transition-metal ions
- Several organic and inorganic molecules
- Biologically relevant molecules, including amino acids, nucleotides and their components
Quantities Obtained from LJ-PES Include:
- Valence (including lowest vertical ionization (VIE) and detachment (VDE) energies) and core-level electron binding energies
from water solvent and atomic and molecular solutes at the solution – vacuum interface as well as from bulk solution - Photoelectron angular distributions to reveal both anisotropy parameters β1 and β2
(here the formula; β2 is zero for non-chiral potential) according to the differential photoionization cross section:

- Auger-electron spectra and detection of autoionization electron spectra resulting non-local (core-level) relaxation processes,
such as Intermolecular/Interatomic Coulombic Decay (ICD), and Energy Transfer Mediate Decay (ETMD) - X-ray absorption spectra via resonant PES (RPES)
Aqueous-Phase Properties and Processes Studied Include:
- Ion pairing
- Oxidation states
- Hydration structure
- Hydrogen bond strengths
- e- mean free paths & scattering
- Water and aqueous solution work functions
- Chemical equilibria at the aqueous solution – vacuum interface
- Solute distributions across the aqueous solution – vacuum interface
- Interfacial species at transition-metal-oxide nanoparticle interface with bulk aqueous solution
- Photo – Auger electron interactions; Post-Collision Interactions, PCI
- Intermolecular charge & energy transfer (from ICD and ETMD)
- Structure, nuclear dynamics, reactive transients, and chemical transformation from core-level relaxation detection (RPES)
Experimental variables exploited in our LJ-PES studies are:
- Photon energy: up to 1600 eV
- Direct Photoemission
- Resonant Photoemission (RPES) with the option to obtain so-called Partial-Electron-Yield X-ray Absorption Spectra PEY-XAS)
- Light polarization: linear and circular
- Liquid microjet: flat and cylindrical (with optional temperature variation)
- Detection angles; PADs
Spectroscopic Methods
Liquid jet photoelectron spectroscopy (LJ-PES) is an excellent tool to probe electronic structures of liquid samples by direct photo-induced electron emission, but the subsequent (ultrafast) electron dynamics induced by ionization also give rise to secondary electrons via electronic decay and auto-ionization. Molecular-level information on electronic structure properties and intermolecular interactions can thus be obtained from the energetic and angular distributions of the detected electrons arising from direct as well as indirect processes.
Binding energies of valence as well as core electrons are sensitive to the local environment surrounding the molecule. Chemical shifts of the orbital energies either with respect to gas phase values or as a function of a solution parameters (pH, solute concentration, temperature, etc.) can thus reveal important information on aspects such as solvation and solvation dependent conformational changes. Solvent effects comprises non-specific electrostatic interactions, specific interaction such as hydrogen bonding, and cooperative effects, and these effects can all influence both conformational and electronic structures and thus have high impact on chemical reactivity.
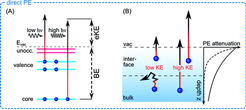
A notable feature of photoelectron spectroscopy is the possibility to sample electronic structure information from different depths into the solution, and thereby to investigate changes in solute densities across the solution interface. This is for example important for atmospherically relevant chemistry (e.g., aerosol chemistry), as surface active solutes can alter surface and interfacial chemistry drastically.
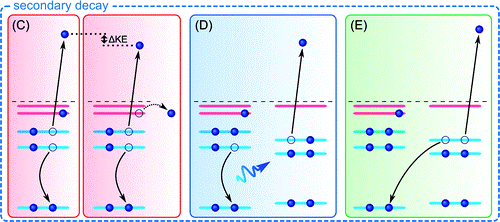
Upon core ionization of a molecule highly excited cations are formed; in the liquid phase both local and non-local (ultrafast) decay processes contribute in a competition that depend on solvation configuration and the nature of the intermolecular interactions that form the immediate solvation shell.
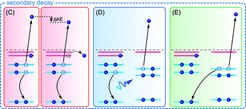
Local Auger decay often dominates over non-local processes; in a conventional Auger-process the core-hole is refilled by a local electron, the relaxation energy is then used to eject another valence electron from the same molecule thus forming a dication. The alternative non-local processes comprise intermolecular Columbic decay (ICD), proton-transfer-mediated charge separation (PTM-CS), and electron-transfer-mediated-decay (ETMD) and all produce ultrafast charge delocalization.