Selectivity in Catalysis
Bukas Group
Welcome to the Catalysis group of the FHI Theory department!
We are working on fundamental understanding of heterogeneous thermal and electro-catalytic processes, with a special focus on product selectivity.
Our research integrates electronic structure calculations with thermodynamic and kinetic modeling, thus aiming to provide predictive-quality simulations of atomic-scale resolution. Among the many challenges that we want to tackle are questions about e.g. the evolving catalyst surface under realistic operating conditions, microscopic mechanisms of (de)activation/poisoning, and the main factors or descriptors that govern catalytic performance. Our models are continuously challenged and tested against well-defined experiments through active collaborations within the new Catalysis Laboratory or 'CatLab' platform (click here for more information on CatLab’s scientific mission and goals).
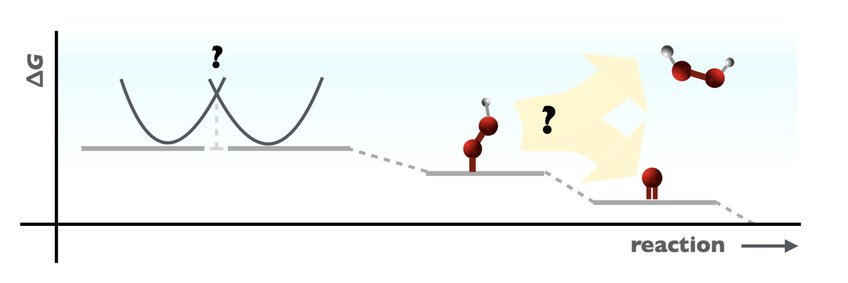
One of the group’s key objectives is to elucidate catalyst selectivity, the origin of which often remains poorly understood especially in the field of electrocatalysis. A characteristic example is the oxygen reduction reaction (ORR) which is known from experiments to proceed either via a four-electron reduction process toward H2O and/or two-electron reduction toward H2O2, depending upon the specific reaction conditions and catalyst in question. The ORR product distribution is not captured by simplified thermodynamic models - despite their many other successes - as these always favor the four-electron over the two-electron route. To answer the question of electrocatalytic selectivity, we work towards a better understanding of interfacial charge transfer reactions: the mechanisms of electron/proton transfer and theoretical modeling of electrochemical barriers.