First-principles Modeling of Solid-Liquid Interfaces and Electrocatalysis
Hörmann Group
Shifting the energy supply towards renewables necessitates the development of energy storage systems, in particular based on catalytic and electrochemical transformations of materials at solid-liquid interfaces such as in batteries or fuel cells. An in-depth, atomic-level characterization of these highly relevant solid-liquid interfaces is crucial for the understanding and improvement of such energy conversion systems. At the same time, electrified interfaces and reactions at those are a challenge for atomistic simulations, due to time and length scales and the involved charge-transfer processes that necessitate quantum-chemical methods for their correct description.
To meet such challenges, we combine in our group a range of methods and approaches to study electrified solid-liquid interfaces under realistic conditions. In particular, we rely mostly on atomistic modelling based on force fields and density functional theory calculations e.g. in implicit solvent environments, which combined with molecular dynamics, statistical sampling, mean field theory and kinetic models allow to derive macroscopic observables in a straight forward way.
Ab-initio based Thermodynamics
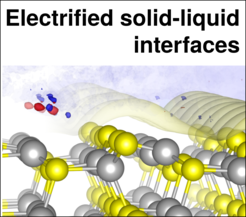
A key research objective is the better understanding of the interfacial thermodynamics. Our research adresses mainly fundamental questions concerning (1) the structure of the solvent at the electrified interface, (2) the explicit application of a potential and (3) the correct thermodynamic treatment of solid-liquid interfaces. For this we work mainly on metallic model systems, but are moving as well towards promising new materials, such as semiconductors and 2D materials.
Furthermore recent advances in the application of an explicit electrode potential in calculations enables to understand and simulate a range of effects that are still poorly understood: Among these are e.g. non-trivial pH and electrolyte effects on Pourbaix diagrams as well as cyclic voltammograms.
Ab-initio based Kinetics
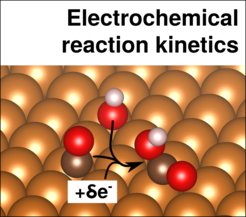
The reaction kinetics at electrified interfaces has been described largely using simplified models. In particular, the most prevalent approach is based on the description of the adsorbate energetics derived from charge-neutral, vacuum or vacuum-like (e.g. with water adlayers) calculations. In addition, very often, electrochemical reaction barriers are left aside and/or determined based on reaction mechanisms inspired by gas-phase catalysis. The correct description of the effect of the electrode potential on the kinetics, e.g. via the variation of electrochemical reaction barriers, is still a challenge.
We are thus starting to look at, implicit/expicit hybrid models which are are promising candidates to solve a range of those issues by natively enabling the explicit application of a potential and the water environment in simulations of reaction barriers in a consistent and computationally efficient way.

