Research
Liquid-vapor interfaces
Aqueous Solution/Vapor Interfaces
The properties of liquid/vapor interfaces play a major role in natural processes. For example, they strongly influence the abundance and reactivity of trace gas molecules that govern heterogeneous processes in atmospheric and environmental chemistry. In particular aqueous aerosols catalyze heterogeneous reactions in the troposphere and can act as both sinks (e.g., HNO3, HCl, N2O5) and sources (e.g., halogen radicals) for atmospheric trace gases. To date, little is known about the concentration of a wide range of solution phase species at the liquid/vapor interface, which can significantly differ from that in the bulk.
Even less is known about the fundamental pathways in heterogeneous reactions of gas phase species at liquid/vapor interfaces. While our knowledge about heterogeneous chemical reactions at solid/vapor interfaces has rapidly expanded over the past decades, the direct measurement of reaction products and mechanisms at the liquid/vapor interface is still hampered by the difficulty of applying surface science techniques to samples with high vapor pressures. Our goal is to develop new experimental tools and platforms to simultaneously measure the surface and bulk chemical composition of aqueous solutions. Emphasis is on monitoring the ion segregation and surfactant layers at the liquid/vapor interface, which lead to a distinctly different chemical composition of the interfacial region from that of the bulk, and to study reactions at the interface and in the bulk as a function of time, which are coupled due to the high diffusion coefficient in aqueous solutions, resulting in fast transport of reaction products from the interface into the bulk and vice versa, as shown in the figure above.
Ice/Vapor Interfaces
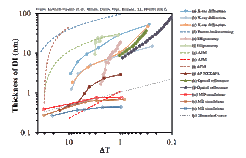
The ice/vapor interface governs many processes, including chemical reactions over the polar icepack and on frozen aerosol particles, and the ease of skiing and skating. One intriguing feature of the ice/vapor interface is the existence of a liquid-like -or disordered (DI) - layer at temperatures close to the melting point. While there is general agreement that this layer will influence the chemical and physical processes at the interface, its basic properties, such as the thickness as a function of temperature, are highly controversial, as the figure below shows, with ΔT as temperature below the melting point. The divergent results are most likely caused by two factors: For one, different methods use different properties of the sample to distinguish between crystalline ice and the liquid-like layer, such as disorder in the oxygen sublattice (proton backscattering, X-ray diffraction) or differences in the optical properties of ice and water (ellipsometry). Another possible reason for the discrepancies seen in the figure above is the presence of contamination, e.g. carbonaceous material or alkali halide ions at the interface, which might increase the degree of premelting. Our approach is to use ambient pressure XPS to monitor the chemical composition of the ice surface (i.e. the presence of contamination) and to utilize near edge X-ray absorption fine structure (NEXAFS) spectroscopy to measure the degree of premelting.

