Biomolecules, Cations and Clusters in the Gas Phase
To understand the function of small molecular systems or larger biological molecules, it is necessary to study and their structure and dynamics. The research in our group is dedicated to the investigation of small modell systems for hydrogen bonding and carbo cations as well as biologically intersting systems involving peptides, proteins and carbohydrates in the gas phase. We use ion mobility-mass spectrometry (IM-MS) to study the overall shape of proteins and carbohydrates and to separate complex isomeric mixtures. Complementary to that, we study the IR signature of our analytes in ultra-cold helium droplets to obtain more detailed insights information on structure and intramolecular interactions of small model systems, carbocations, and peptides or even proteins. To learn more about current research see our recent publications.
Mass-selected ions in liquid helium droplets
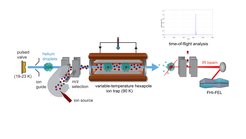
Liquid helium droplets are ultra-cold and superfluid and thus provide a unique environment for molecular species. In most experiments, however, droplets can only be doped with molecules that can be thermally evaporated, thus excluding interesting species such as larger biomolecules.
We developed a machine that allows the investigation of mass-selected clusters or biomolecular ions in liquid helium droplets. The droplets are generated by a pulsed valve, kept at cryogenic conditions. They then traverse an ion trap, filled with mass-selected ions. Upon impact of a droplet on an ion, the ion can be embedded into the droplet and because of the high kinetic energy of the droplet (a result of its high mass), the doped droplet can leave the trap to be further investigated.
High resolution ion mobility spectrometry, combined with optical spectroscopy
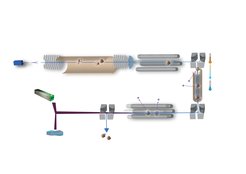
In ion mobility spectrometry (IMS), (biomolecular) ions drift through a buffer gas under the influence of a weak electric field and are separated according to their size, charge and shape. By employing this technique isomers and conformers be separated. In addition, the ion's angular averaged collision cross section (CCS) can be calculated from mobilities and compared to structures obtained via molecular modelling.
Gas-phase spectroscopy on the other hand, provides direct information about the underlying structure of the ion. Vibrational spectroscopy for example, is highly sensitive to the presence of hydrogen bonds and, therefore, yields information about the underlying secondary structure of the molecule.
Here, we are combining both orthogonal methods to elucidate the gas-phase structure of conformer-selected proteins and their non-covalent assemblies. Therefore, we constructed a high-resolution IMS apparatus which integrates with a cryogenic ion trap to facilitate spectroscopic experiments. Tunable infra-red radiation for these experiments is provided by the FHI free-electron laser.

