Understanding X-ray Radiation Damage
A groundbreaking study involving the Fritz Haber Institute in Berlin, the University of Kassel, the University of Heidelberg, and Uppsala University has unveiled the mechanisms by which X-rays cause damage in liquids. Published in Nature Communications, these findings could lead to safer radiotherapy and X-ray examinations.
Key Findings
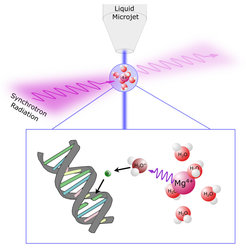
In the study titled “X-ray radiation damage cycle of solvated inorganic ions,” scientists exposed a magnesium salt solution to X-rays. Magnesium salt, prevalent in the human body, is vital for nerve function and certain DNA proteins. The research team used it to trigger cyclic radiation damage, as detailed in their paper.
X-rays, characterized by low wavelength and high energy, produce water radicals and low-energy electrons in the magnesium salt solution. These particles are known for their mutagenic effects, such as causing double-strand breaks in DNA.
Unique Radiation Cycle
What sets this cycle apart is that the dissolved magnesium ions, despite their initial excitation, revert to their original state after producing these particles. This allows the damaging release of electrons to repeat in a localized area, similar to repeatedly hammering the same spot.
Implications
Understanding these processes is crucial. Enhanced knowledge can lead to significant improvements in the safety and effectiveness of X-ray and radiation therapy devices.
Collaborative Effort
The international team conducted their experiments at X-ray radiation sources in Hamburg (DESY) and Berlin (BESSY II), showcasing the power of collaborative research in advancing scientific understanding.












