Discovery to lower CO2 Emissions in the Industrial Sector by using Green Synthesis
Achieving a net zero greenhouse gas balance is a major challenge facing the chemical industry. The goal can be met by reducing the energy demand of chemical processes and the sustainable use of raw materials. Researchers of the Fritz-Haber-Institute have shown that the valuable intermediates propylene and propylene oxide can be formed directly by oxidation of propane in the gas phase over nonspecific interfaces, such as sea sand, without producing significant amounts of CO2.

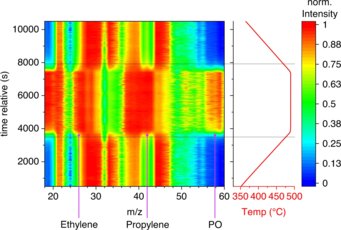
The chemical industry is responsible for around 14% of CO2 emissions in the industrial sector. Catalysis research plays a key role in reducing these emissions. The catalyst can directly prevent the formation of unwanted carbon dioxide or reduce the energy required for a reaction and/or downstream processing of the products. However, complex catalyst optimization does not always lead to success. We have found that a fast reaction at nonspecific interfaces can be used very effectively for the synthesis of fragile products that need to be synthesized by oxidation and that are readily further combusted to carbon dioxide in the presence of a catalyst. The valuable intermediates propylene and propylene oxide can be formed directly by oxidation of propane in the gas phase over inert filling materials, such as boron nitride, silicon carbide, or sea sand, without producing significant amounts of CO2. Although propylene oxide is known as an intermediate product of the combustion of propane with molecular oxygen, for example from model studies of combustion processes in engines, the formation of propylene oxide from propane and oxygen has never been reported in oxidation catalysis. Using mass spectrometry, it was possible to prove that self-ignition is necessary for the formation of the products. Researchers from the Theory Department of the FHI confirmed that the reaction occurs in the gas phase and is promoted by OH radicals.

The finding is of fundamental importance for oxidation catalysis research. In heterogeneous catalysis, the exclusive formation of a desired product usually requires a highly specific interaction of the reacting molecules with the catalyst. However, strong interaction leads to long residence times of the intermediates on the surface, which can cause subsequent reactions and in oxidation catalysis to the total combustion of valuable resources to undesired carbon dioxide. Therefore, it is not the optimization of the catalyst but the optimization of the adsorption-desorption kinetics that is important if fragile products such as propylene oxide are of interest.
The discovery also points the way to environmentally friendly production of propylene oxide and propylene in a single step. Propylene is currently produced on a large scale by thermal cracking as a feedstock for polymerization. Propylene oxide is a major intermediate in the chemical industry for synthesizing a large variety of consumer products including polyether polyols that are used in the manufacture of polyurethanes, propylene glycols as raw materials for the production of unsaturated polyester resins applied in the textile and construction industries, and propylene glycol ethers utilized as solvents in paints, inks, coatings and many other related applications. The need of expensive auxiliary chemicals such as hydrogen peroxide, the complexity of the processes, and considerable environmental burdens due to waste formation imply economic drawbacks of the current multi-step production technologies of propylene oxide. Process simulations in collaboration with the Max Planck Institute of Chemical Energy Conversion in Mülheim an der Ruhr demonstrated that the combined synthesis of the two important chemical building blocks propylene and propylene oxide in the simple direct oxidation of propane over inert filling materials is indeed technologically feasible.