Selectivity of Catalysts
In order to successfully shape the energy transition and achieve CO2 neutrality in society, it is important to develop technologies through which the greenhouse gas CO2 can be efficiently converted into chemical raw materials and fuels. The electrochemical CO2 reduction reaction can fulfil this task by using electricity from renewable sources.
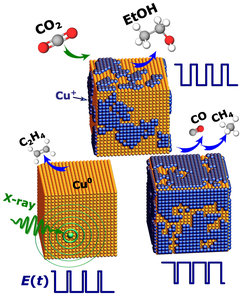
However, the question of how the reaction products can be controlled and managed has remained unanswered until now. Researchers from the Department of Interface Science at the Fritz Haber Institute and Christian Albrechts University in Kiel have now found that this is possible by applying a periodic electrical voltage (voltage pulses).
The electrochemical CO2 reduction reaction (CO2RR) is a cost-effective and environmentally friendly way to convert CO2 into valuable chemicals and fuels when powered by renewable energy and supported by CO2 catalysts. However, the range of possible reaction products is broad and the correlations to the different catalyst properties, especially during the reaction, are poorly understood. This hinders the practical application CO2RR. The research team has now extensively explored the properties of the electrocatalyst under reaction conditions and shown that the structure of the catalyst can be controlled as needed by dynamic reaction conditions. With this idea, they were able to systematically investigate the relationship between the catalyst structure and the catalytic properties and develop a recipe for controlling the selectivity with respect to specific chemicals. The research results were published today in Nature Catalysis.
Copper-based catalysts, especially those derived from copper oxide, have long shown promising selectivities with respect to high-value hydrocarbons under constant voltage. Nevertheless, controlling the selectivity of the catalysts remains a major challenge due to the complex reaction mechanisms of CO2RR. By using size- and shape-controlled Cu2O nanocatalysts, the researchers have shown that the critical parameters affecting the properties and function of the catalysts can be conveniently adjusted. For example, the oxidation state of copper and the structure of the oxide formed can be controlled by an appropriate choice of the applied voltage and by the temporal duration of the voltage pulse. In particular, the balance between oxidised and metallic copper on the catalyst surface and directly below can be maintained over sustained reaction times by a combination of short anodic (oxidative) and cathodic (reductive) voltage pulses. An important achievement of this study is the favourable production of ethanol due to the obtained dynamic equilibrium between oxidised and reduced copper species. This allows a dramatic increase in the CO2 selectivity of ethanol compared to static CO2RR conditions.
Furthermore, a highlight of this work is the demonstration that structural changes of the catalyst under operando reaction conditions can be followed by using time-resolved synchotron-based methods. More specifically, the FHI researchers were able to observe the periodic changes in the copper oxidation state and thereby discovered the formation of a distorted oxide structure that is regenerated after each postential pulse. These concrete changes in the chemical and geometric structure of the catalyst can explain the observed improvement in selectivity trends, in which a metallic copper surface favours ethylene production, while Cu2O mainly promotes methane and CO. However, the simultaneous presence of the distorted copper oxide as well as metallic copper facilitates ethanol production. These results were obtained by a synergistic combination of X-ray absorption spectroscopy, X-ray diffraction and X-ray photoelectron spectroscopy.
The study demonstrates the enormous potential of pulsed electrolysis to control and optimise catalyst performance and highlights the importance of operando studies - a focus of the Interfacial Science Department - in understanding a new generation of catalysts.












