SOx on Ag catalysts and its role in alkene epoxidation
Emilia A. Carbonio, Alexander Yu. Klyushin, Frederic Sultzmann, Michael Hävecker, Simone Piccinin, Axel Knop-Gericke, Travis E. Jones, Robert Schlögl
The direct partial oxidation of ethylene to ethylene oxide (EO) over Ag highlights the tremendous potential of heterogeneously catalyzed partial oxidation reactions. Ag catalysts can be made to favor ethylene epoxidation by ca. 90% over the thermodynamically favored total combustion, but this performance is not ubiquitous. The Ag catalysts that are so useful for EO production fail dramatically when used to produce another important feedstock epoxide, propylene oxide (PO). This failure may appear surprising owing to the structural similarity between EO and PO, yet extensive studies of Ag-based catalysts in the direct epoxidation of propylene demonstrate Ag favors total combustion. Thus, PO is currently mainly produced by either environmentally unfriendly or costly process.1 We have recently identified the nature of the oxygen species that can produce EO on Ag catalysts. This oxygen originates from adsorbed SO4 (SO4,ads) which is part of a non-stoichiometric two-dimensional Ag/SO4 phase where the sulfur is formally S(+V). 2 On a surface free of oxygen SO4 forms an unreactive SO4(7 × √3)rect surface reconstruction. When oxygen induced surface reconstructions are formed on such a surface, however, the Ag/SO4 reconstruction is partially lifted resulting in the formation of SO4,ads. TPR experiments and DFT calculations show SO4,ads can produce EO.2 NAP- XPS experiments demonstrated that the EO selectivity tracks the coverage of SO4,ads, and that SO4,ads is present on the Ag surface for steady state ethylene epoxidation.2 A significant population of SO3,ads is only observed under a pure ethylene atmosphere, when complete titration of SO4,ads occurs due to the absence of the adsorbed O necessary to sustain the SO4,ads population by re-oxidation of SO3,ads.2
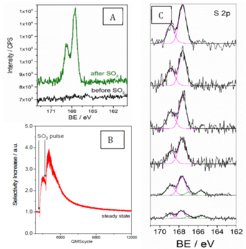
Following this result, we used NAP-XPS to study the Ag surface under propylene epoxidation conditions. We find that as opposed to ethylene epoxidation, SO4,ads is not present under steady state propylene oxidation conditions (Figure 1a). SO4,ads can, however, be formed by introducing an SO2 pulse to the reaction feed, resulting in an increase in selectivity to PO (Figure 1b). However, SO4,ads is rapidly titrated under reaction conditions and PO selectivity decreases with time following the decrease in SO4,ads coverage. During this process we observe the formation of SO3,ads (Figure 1c). As for ethylene epoxidation, it seems that SO4,ads is also responsible forpropylene epoxidation and SO3,ads is seen as a titration product. However, NAP-XPS demonstrates atomic O has a low coverage under propylene epoxidation conditions compared to those for ethylene epoxidation. As a consequence, SO4,ads is continuously titrated under propylene epoxidation conditions, resulting in a low steady state coverage. In addition, low coverage of adsorbed atomic O precludes the formation of oxygen induced surface reconstructions3, necessary to partially lift the Ag/SO4 reconstruction and make the active species SO4,ads. It appears that the O coverage on Ag has a critical role in mediating the coverage of the active species SO4,ads under steady state conditions. The co-existence of SO4 and atomic O on the Ag surface appear to dictate the (high) EO and (low) PO selectivity.
References
1. Khatib, S. J.; Oyama, S. T., Direct Oxidation of Propylene to Propylene Oxide with Molecular Oxygen: A Review. Catalysis Reviews 2015, 57 (3), 306-344.
2. Jones, T. E.; Wyrwich, R.; Bocklein, S.; Carbonio, E. A.; Greiner, M. T.; Klyushin, A. Y.; Moritz, W.; Locatelli, A.; Mentes, T. O.; Nino, M. A.; Knop-Gericke, A.; Schlogl, R.; Gunther, S.; Wintterlin, J.; Piccinin, S., The Selective Species in Ethylene Epoxidation on Silver. Acs Catal 2018, 8 (5), 3844-3852.
3.Carbonio, E. A.; Rocha, T. C. R.; Klyushin, A. Y.; Pis, I.; Magnano, E.; Nappini, S.; Piccinin, S.; Knop- Gericke, A.; Schlogl, R.; Jones, T. E., Are multiple oxygen species selective in ethylene epoxidation on silver? Chemical Science 2018, 9 (4), 990-998.
