From greenhouse gas to fuel: dynamically-driven Cu surfaces catalyze the electrochemical reduction of CO2 to ethanol
Renewable power sources with zero emissions such as solar and wind contribute significantly to the satisfaction of our energy demands, although their intermittent nature (the sun doesn’t always shine and the wind doesn’t blow all the time) cannot supply the energy that is required on a daily basis. However, this energy could be stored for later use through the electrocatalytic reduction of CO2.
The idea behind the CO2 electrochemical reduction is to store this intermittent energy in molecular bonds, i.e. to covert CO2 into chemical feedstocks and fuels that can later on be employed in classical combustion or in a fuel cell to produce energy. In this way we can also close the anthropogenic carbon cycle and avoid further emissions to the atmosphere.
To address this challenge and achieve high activity and selectivity, the hunt for new catalysts and the fundamental understanding of this reaction are of paramount importance. Structural and compositional effects have been widely studied for this reaction, and especially the catalytic role played by Cu(I) species has been a topic under strong debate within the catalysis community.
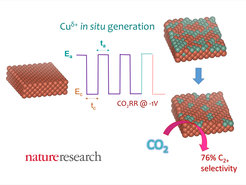
The team around Dr. Rosa Arán-Ais, including Fabian Scholten, Sebastian Kunze, and Dr. Rubén Rizo, that works under the direction of Prof. Beatriz Roldán Cuenya in the Department of Interface Science at the Friz Haber Institute have provided an unprecedented experimental proof of the synergistic effect of specific structural motifs on Cu surfaces together with Cu(I) species in the C―C coupling pathway, more specifically, towards the production of ethanol.
Significantly enhanced selectivity for ethanol was found at -1.0 V under pulsed electrolysis conditions where Cu(I) and special structural motifs were continuously in situ (re)-generated. This finding was assessed by the combination of ex situ and in situ electrochemical, microscopic and spectroscopic techniques.
The fundamental understanding created by this study is extremely valuable for rationally tailoring electrochemical interfaces with constrained surface structure and tunable composition towards selective C2 product production.












