Watching catalysts at work
With climate change and global warming being amongst the biggest problems we are facing today, scientists are increasingly researching ways of producing green energy. ‘Green’ in this case means that this type of energy has a low or, ideally, no carbon footprint. Solar and wind energy are good examples where this was achieved. But as great as they are, they do not satisfy all the energy needs we have. While they may provide our electric sockets in our homes with energy, they cannot replace liquid fuels we need for travel and transport, because they cannot be stored in an easily accessible way.
For this reason, scientists are trying to produce green liquid energy sources, and they have found a pretty spectacular way of doing it. For a while now, they have known that CO2 – the greenhouse gas we’re trying to get rid of because it promotes global warming – can be transformed into an energy source. One of the process that can be employed for this is the “electrochemical reduction of CO2 (CO2RR)”, whereby small CO2 molecules are converted into alcohols and hydrocarbons that are stable for later use – analogous to how plants convert CO2 into carbohydrates and sugars during photosynthesis.
“This strategy is particularly appealing because using alcohols as fuels for combustion is highly compatible with our current energy infrastructure”, explains Prof. Beatriz Roldán Cuenya, Head of the Interface Science Department, where the electrochemical reduction of CO2 is studied closely. “If the energy needed for this is already green (i.e. comes from wind or solar power), then there is no carbon footprint and we can close the anthropogenic carbon cycle, thus limiting further CO2 emissions to the atmosphere”, she adds enthusiastically.
While this idea sounds great, the production of liquid fuels from CO2 is complicated. Dr. See Wee Chee, Group Leader at the Interface Science Department, explains why: “The production of the compounds such as alcohols and useful hydrocarbons from CO2 needs an electrocatalyst, a substance that makes the reaction happen faster or with less energy. But CO2RR is too often hampered by poor activity, selectivity and stability of the electrocatalysts to make it economically viable.” Scientists such as those at the Department of Interface Science are constantly trying to design better catalysts, but that, too, is not easy. The main problem is that it is not well known what these catalytic structures are like during the reaction. “We’re looking at catalysts like detectives sorting through evidence from photographs. You know the scene before and after the ‘crime’, and from there you deduce what happened in between. What you really want, though, is a video of what happened,” explains Dr. Chee. It is crucial to gain a fundamental understanding of how the catalysts are changing during reaction in order to design better ones. Unfortunately, the available techniques for observing such reactions do not provide the necessary resolution.
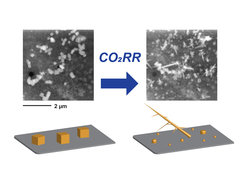
Researchers at the Interface Science Department and the Department of Inorganic Chemistry have now employed a new way of solving this problem. The team involving electron microscopists and electrochemists used a technique called “liquid cell transmission electron microscopy” (TEM) to study the behavior of catalysts for the CO2RR reaction. While this technique has been around for some time, the research group at the FHI is one of the first groups to try it in this context. The challenge is that the copper catalysts, which are very small, have to be placed into tiny liquid reaction cells that have volumes less than a nanoliter (a million times smaller than a liter). The researchers found a clever solution for this problem: they synthesized the copper cubes (Cu2O) inside the liquid cells using an electrochemical recipe they previously patented. They basically grew the catalysts directly where they could observe them under CO2RR conditions. They found out that the catalyst, as they suspected, did not look the same as it did before the reaction, instead, it changed very rapidly.
These studies show that highly detailed information about catalyst structures under electrochemical reaction conditions can be obtained using liquid cell TEM. Furthermore, the lessons learnt from looking at how the copper catalyst changed also help scientists understand better how to control it. Moving forward, the Liquid Electron Microscopy group led by Dr. See Wee Chee aims to use this technique to gain in-depth insight into structure-property correlations in electrocatalytic materials. This will further advance technologically important reactions for sustainable energy such as water splitting (a reaction in which water is broken down into oxygen and hydrogen) for green hydrogen production.












