The role of Oxygen States in NiO for Oxygen Evolution Reactions
Raoul Blume, Wolfram Calvet, Aliakbar Ghafari
In recent years NiO has come into focus as a low-cost, efficient material in the Oxygen Evolution Reaction (OER) via electrochemical water splitting. However, the detailed mechanism of the electrochemically induced reaction is not yet fully understood. In order to understand the details of OER mechanism of NiO, XPS and XAS techniques for two types of experiments were used. Firstly, as a model catalyst, NiOx thin films were put in contact with oxygen and subsequently water vapor at 0.5 mbar and elevated temperatures. Secondly, in operando electrochemical experiments were performed on thin NiOx films. We are able to show that in the reactions oxygen vacancies are formed and seemingly play a crucial role acting as an intermediate state in OER.
NiO model catalyst
Thermal treatment of NiO films in O2 reveals the formation and replenishing of oxygen defects in dependence of the sample temperature as evidenced by the alternating oxygen content of the sample (Fig. 1 c). Such defects are detected as a distinct broadening of the main NiO O1s feature at 529.7 eV as well as a reversible intensity change of the peak correlated with Ni vacancy sites at 531.4 eV (Fig. 1a). DFT calculations of the relaxed NiO lattice connect the observed O1s broadening to changing bond lengths around vacancy sites. Corresponding Ni2p and VB spectra exhibit a correlated, reversible change most likely caused by non-local screening effects (Fig. 1b). Subsequent heating-cooling in H2O vapor suggests that oxygen defects help to instigate H2O dissociation leading to metastable OH chemisorption at the defect sites. Repeated heating-cooling slowly transforms the NiO surface into stable Ni-OOH as evidenced by a strong intensity gain in the respective binding energy region (530 – 534 eV) and a distinct increase of the oxygen content (; Fig. 1d & e). Hence, oxygen defects not only acts as a precursor to OH-bond formation but may act as an intermediate in OER.

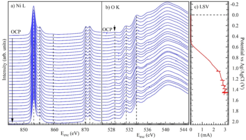
Figure 2a and b show XAS spectro-electrochemistry of NiO respectively, for Ni L-edges and O K-edge which have been measured during linear sweep voltammetry (LSV) from 0 to 1.5 V (vs. Ag/AgCl) in 0.1M KOH electrolyte (pH 13) using three electrode method. The Ag/AgCl electrode has been used as a reference electrode. The spectra at the top in figure 2a and b, are related to the open circuit potential (OCP). It can be observed that the intensity of pre-peak of O K-edge at 528.5 eV is increased by increasing of potential while Ni L-edge do not show such a development of peak.