New Insights into Green Hydrogen Production and Beyond
The Interface Science Department of the Fritz Haber Institute has made significant strides in understanding the critical process of interfacial ion solvation, as detailed in their latest publication, “Ion Solvation Kinetics in Bipolar Membranes and at Electrolyte-Metal Interfaces" in the prestigious journal Nature Energy.
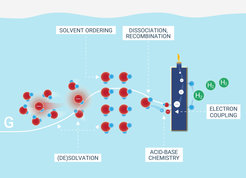
This discovery sheds light on a process that is ubiquitous across bio- and electrochemistry: Ions need to reorganize their solvation shell before they can intercalate into battery cathodes, enter ion channels across biochemical membranes or adsorb and convert to chemicals on electrocatalyst surfaces. Conversely, this process is key for the production of green hydrogen, and thus offers new perspectives for chemical conversion technology.
Central to this discovery is the understanding of how water molecules dissociate and how ions, such as protons and hydroxides, are solvated at reactive interfaces. The team's research, conducted at polymeric bipolar membrane junctions and selected electrocatalyst interfaces, reveals surprising findings about the relationship between the electrochemical potential, activation entropy, and energy.
Contrary to long-standing assumptions in electrochemistry, the team found that increasing the electrochemical bias does not primarily reduce the activation energy but instead increases the activation entropy. “This is opposed to what you are taught in a typical electrochemistry course.” says Carlos Gomez Rodellar, first author of the study and PhD student in the Interface Science Department. “The common view is that the electrochemical bias primarily reduces the activation energy.”
Dr. Sebastian Öner, Group leader at the Interface Science Department and corresponding author of the study, emphasizes the importance of these findings not only for electrocatalysis and bipolar membranes but also for applications in bio- and thermal catalysis. „ Our fundamental results imply that excess capacitive charge and electric fields directly impact the entropy of the transition state. This discovery will allow us to explore new science and technology applications." says Dr. Öner.
This research highlights the importance of the local environment of catalysts, showing how the solid structure and liquid electrolyte are closely interconnected and can influence each other. This comprehensive understanding is crucial for developing catalysts with improved activity, selectivity, and stability.
The Interface Science Department, led by Prof. Dr. Beatriz Roldán Cuenya, is committed to further exploring these insights, with the potential to significantly impact the fields of energy and chemical conversion technology.












