Differentiation of strongly bound oxygen on silver
F. Sulzmann, E.A. Carbonio, T. Jones, A. Klyushin, A. Knop-Gericke, R. Schlögl
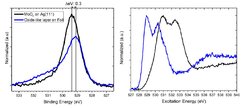
Understanding the catalytic partial oxidation of methanol to formaldehyde is a long standing problem that is poised to face a resurgence in interest. Nowadays, formaldehyde is produced industrially by the reaction of methanol over either metal-oxide or silver based catalysts. The two catalysts require different reaction conditions and show different selectivity. For example, it is believed that formaldehyde is produced through both a partial oxidation and dehydrogenation pathway over silver based catalysts, while only the former seems to be important for the metal-oxide catalyst.1 The existence of multiple pathways on silver is a particularly interesting problem as they give different co- products—H2O or H2 for the partial oxidation and dehydrogenation pathway, respectively. Early ex situ studies suggested the two pathways can be attributed to the presence of different adsorbed oxygen species,2 yet other evidence leads to contradictory claims.3 Our work is motivated by resolving the controversy surrounding the nature of the oxygen species present on silver based catalysts during the catalytic partial oxidation of methanol and determining their roles in the reaction. The silver oxygen system has been thoroughly investigated in the last decades as it is the basis of important large scale industrial processes such as ethylene epoxidation and methanol oxidation. The identification of different oxygen species was originally carried out by way of TPD measurements. Rehren et al.4 were the first to identify three different oxygen species with the help of isothermal and isobaric TPD measurements. In addition to the already identified adsorbed atomic oxygen (Oα) , and bulk dissolved oxygen (Oβ), they found a third oxygen species stable up to high temperatures [675-710 °C] which they denoted Oγ. These results were then correlated with XPS performed in ultra-high vacuum (UHV) and later NAP- XPS.5 Here, the notation and correlated O1s binding energies form Rocha et al.5 were used. Due to its thermal stability the oxygen species of interest for the methanol oxidation is Oγ, with a binding energy of 529.1 to 529.7 eV.5 It is described as a thin oxide-like-layer on the silver surface. Since at the relevant temperatures silver undergoes faceting to expose Ag (111), Oγ is thought to form only on the (111) facet. Rocha et al.5 went on to show two oxygen Figure 1: a) O1s spectra of oxide-like layers on Ag foil and MoOx on Ag(111) surface measure at 500 °C in 0.25 mbar O2. b) the, corresponding XA spectra a) b)species form at 500 °C in 0.25 mbar of oxygen, Oγ and a second present only under an oxygen atmosphere, which they denoted Oα2. Reichelt et al.6 later used UHV techniques to show the strongly bound Oγ species could, in some cases, be a molybdenum oxide on the silver surface, but the question remains as to the generality of that finding under in situ conditions. Figure 1 shows the O1s XP and O-K edge XA spectra of molybdenum oxide and oxide-like layers grown on a Ag(111) crystal. The small differences of 0.3 eV in binding energy illustrate the difficulties of distinguishing these species using lab based UHV set-ups. More insights can be gained with synchrotron based NAP-XPS set-ups. With this it is possible to see the differences in the O K-edge and investigate the stability in different atmospheres (e.g. MoOx is stable at 500 °C in UHV, the oxide-like layer isn ́t). When coupled with DFT and computational spectroscopy these results allow us to assign Oα2 to thin pure AgOx oxide-like layers.7 At 500 °C in 0.25 mbar O2 we detect a linear correlation between the Oγ species and the molybdenum content on the surface (O/Mo = 2.3). In addition, the comparison of the reported valance band5 and measurements on valance band of MoOx on silver suggest that molybdenum was present on the surface, which would allow an assignment of Oγ to molybdenum oxide. Furthermore, this technique allows us to investigate the molybdenum oxide (and Oα2) under conditions relevant for methanol oxidation. By changing the molar feed ratio from oxygen rich to oxygen lean conditions, differences in the oxidation state of the molybdenum are detectable. This clearly shows that molybdenum oxide is present in different forms depending on the reaction feed.
| Oxygen Species | Binding Energy (eV) | Structure | Reference |
|---|---|---|---|
| O α1 | 528.1-528.4 | Adsorbed oxygen on a p(4x4) phase | Jones et al. [8] |
| O α2 | 529.1-529.2 | Oxide-like layer | This work |
| O γ | 529.5-529.7 | MoO x | This work |
| O α3 | 530.2-530.4 | Adsorbed SO 4 | Jones et al. [7] |
| O β | 530.8-531.0 | SO 4 on (7x√3)rect reconstrucƟon | Jones et al. [7] |
The remaining question is, which of the high temperature oxygen species contribute to reported dehydrogenation pathway? It could be that the confusion in literature arises from some groups having studied MoOx on silver and others the pure AgOx oxide-like layers.
References:
[1] G. Reuss et al. Ullmann’s Ency- clopedia of Industrial Chemistry, fifth ed., A11 (1988)
[2] A. Nagy et al. al. Applied Catalysis A 1-2 (1999) 188.
[3] G.Waterhouse et al. Applied Catalysis A 1 (2004) 265.
[4] C. Rehren et al. Catalysis Letters 3-6 (1991) 11.
[5] T. Rocha et al. Phys. Chem. Chem. Phys. 14 (2012) 14.
[6] R. Reichelt et al. J. of Physical Chemistry C 35 (2011) 115.
[7] T. Jones et al. ACS Catalysis (2018).
[8] T. Jones et al. Phys. Chem. Chem. Phys. 14 (2012) 17.
