Spectroscopic investigation of proton bonding at sub-kelvin temperatures
- MP Department Seminar
- Date: Feb 7, 2025
- Time: 09:30 AM - 10:30 AM (Local Time Germany)
- Speaker: America Torres-Boy
- FHI, Molecular Physics Department
- Location: Building K, Haber-Villa, Faradayweg 8, 14195 Berlin
- Room: Seminar Room
- Host: Department of Molecular Physics
- Contact: torres@fhi.mpg.de
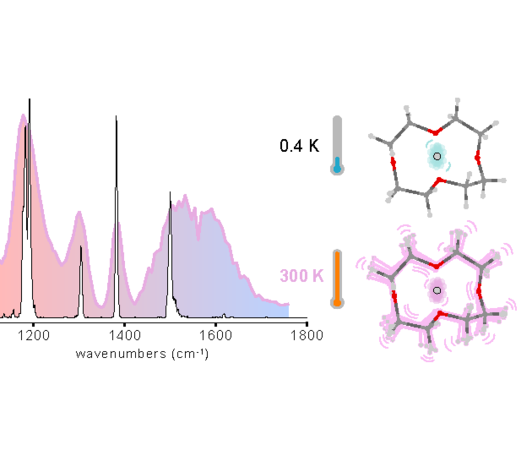
The proton bond is a pivotal chemical motif with significant implications across science and technology, yet its quantum chemical description is challenged by nuclear and charge delocalization effects. This study seeks insights into proton bonding at sub-kelvin temperatures, exposing intrinsic features of the proton bond free from thermal fluctuations of the molecular frame. A proton is bound within the molecular ring cavity of the 12-crown-4 ether, and the resulting ion is isolated in a helium droplet at approximately 0.4 K, where it is examined using infrared laser spectroscopy. The resulting spectra reveal narrow vibrational bands, indicating a robust proton bond bridging ether sites across the cavity of the essentially frozen crown ether. The potential energy surface sustaining the proton bond is broad and markedly anharmonic, causing common modeling methods within the harmonic approximation to fail in capturing the observed band positions. Calculations and measurments at room temperatures show that the crown ether backbone is highly fluxional and that the distance between the oxygen atoms fluctuates in time, modulating the potential that the proton or deuteron is exposed to, and yielding dynamic inhomogeneous broadening and blue shifts with respect to the cryogenic spectra. These observations call for novel computational developments, for which the vibrational signatures outlined in this work should provide a valuable benchmark. These findings underscore the need for novel computational methods, with the vibrational signatures providing a benchmark for future developments.