Electrolyte-induced Nanostructuring Greatly Promotes C-C coupling in CO2 Electrochemical Reduction
The electrochemical production of fuels and chemical feedstocks from CO2 and water using electricity derived from renewable energy holds promise as a sustainable process to mitigate some of the energy and climate challenges. To date, copper is the only metal found promising for CO2 electroreduction towards highly desirable (C2+) products like alcohols and hydrocarbons in considerable amounts. However, pure Cu electrodes (e.g., foils) show poor selectivity towards C2+ products and suffer from the need to apply high overpotentials.
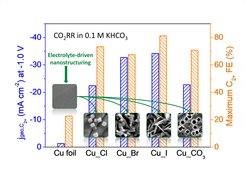
The team of Dr. Gao and Prof. Roldan Cuenya from the Department of Interface Science proposed an elegant and efficient approach, namely, electrolyte-driven nanostructuring, for facile synthesis of highly selective Cu-based catalysts. The approach is based on the electrochemical modification of a Cu surface by cycles of oxidation and reduction in different electrolytes. The initially smooth surface strongly reconstructs, thus forming a layer or a film of Cu nanoparticles having well-defined shape and dimension dependent of the electrolyte used (KCl, KBr, KI, and K2CO3). For instance, the KCl-induced modification results in the formation of Cu nanocubes, while iodide leads to a needle-like morphology. The nanostructured catalysts suppress methane formation but substantially improve selectivity towards ethylene and other C2+ products as compared to the initially flat Cu foils.
To shed light on the promotional effect of halide-modified Cu catalysts, operando High Energy Resolution Fluorescence Detected X-ray Absorption Near Edge Structure (HERFD-XANES) measurements were carried out at the European Synchrotron Research Facility (ESRF). The technique is very sensitive to the chemical state and coordination environment of Cu, allowing the detection of residual Cu+ species under reaction conditions. Among the halide-modified Cu catalysts examined, the KI-modified one showed the highest Cu+ content and the highest Faradaic efficiency for C2+ products. The results revealed a correlation between the production of C2+ and the amount of Cu+ species in the halide‐modified Cu catalysts in the following order: CuI> CuBr > CuCl. Moreover, long-term reactivity studies showed decreased ethylene formation and increased methane production in time which is accompanied by a gradual depletion of the Cu+ species. All these findings provide compelling evidence for that surface Cu+ species adjacent to Cu0 are active sites for C-C coupling.
Although the specific reaction pathways must be further investigated in close collaboration with theory, the work of Gao et al. provides new important insight into the parameters that can be tuned for the rational design of C2+-selective CO2 electroreduction catalysts.












